
Safety of Qfitlia™(fitusiran)
The safety profile of Qfitlia
Most common adverse reactions in people treated with antithrombin-based dosing regimen
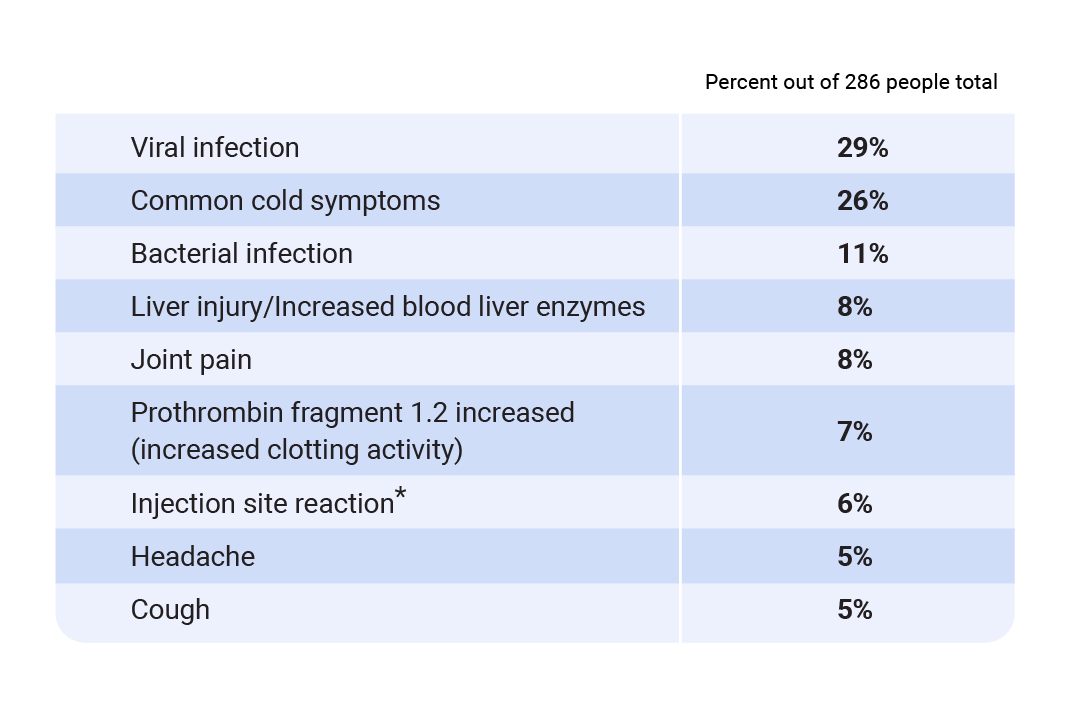
* Redness, tenderness, pain, swelling, warmth, bruising, bleeding, skin changes, rash, or itching at the site of injection.
Your doctor will closely monitor you for certain adverse reactions:
- Abnormal blood clotting (thrombotic events): Serious blood clots have happened in people treated with Qfitlia. Qfitlia can cause blood clots to form in blood vessels in your arms, legs, lungs, heart, brain, eyes, or head. Your risk of blood clots is greater if your antithrombin blood level is persistently less than 15% or if you have certain other conditions. Get medical help right away if you get any of these signs or symptoms of blood clots during or after treatment with Qfitlia: swelling, pain, or redness in arms or legs; coughing up blood; shortness of breath; severe chest pain or tightness of the chest; fast heart rate; feeling faint or passing out; severe or persistent headache; difficulty speaking or understanding language; feeling confused; numbness or weakness in your face, arms, or legs; sudden loss or changes in your vision, eye pain, or swelling. Your doctor may modify your dose based on your antithrombin activity to reduce your chance of blood clots
- Gallbladder disease: Qfitlia can cause gallstones (cholelithiasis) and inflammation of your gallbladder (cholecystitis), which might require surgery to remove your gallbladder. Tell your healthcare provider right away if you develop stomach pain, indigestion, nausea, or vomiting. Your healthcare provider may temporarily or permanently stop your treatment with Qfitlia if you develop any of these symptoms
- Increased liver enzymes: Qfitlia can cause an increase in your blood liver enzymes. Your healthcare provider will do blood tests to check your liver function before and during treatment with Qfitlia
Please discuss any safety questions with your doctor
It is not known if Qfitlia is safe and effective in children younger than 12 years of age.
Qfitlia can cause SERIOUS SIDE EFFECTS, including:
Abnormal blood clotting (thrombotic events): Serious blood clots have occurred in people treated with Qfitlia. Qfitlia can cause blood clots to form in the blood vessels in your arms, legs, lungs, heart, brain, eyes, or head. Your risk of blood clots is greater if your antithrombin (AT) blood level is persistently less than 15% or if you have certain other conditions. Your healthcare provider (HCP) will check your AT blood levels before and during treatment with Qfitlia
Gallbladder disease: Qfitlia can cause gallstones and inflammation of your gallbladder, which might require surgery to remove your gallbladder. Tell your HCP right away if you develop stomach pain, indigestion, nausea, or vomiting. Your HCP may temporarily or permanently stop Qfitlia if you develop any of these symptoms
What is the most important information I should know about Qfitlia?
Qfitlia helps your blood form clots. Do not stop using Qfitlia without talking to your HCP. If you miss doses or stop using Qfitlia, you may no longer be protected against bleeding.
Use of a clotting factor concentrate (CFC) or bypassing agent (BPA) to help protect against bleeding must be stopped within 7 days after your first dose of Qfitlia.
Your HCP may prescribe on-demand CFC or BPA if you bleed during treatment with Qfitlia. Carefully follow your HCP’s instructions regarding when to use on-demand treatment with CFC or BPA, including the prescribed dose and timing of the CFC or BPA.
Get medical help right away if you get any of these signs or symptoms of blood clots during or after treatment with Qfitlia:
- Swelling, pain, or redness in arms or legs
- Coughing up blood
- Shortness of breath
- Severe chest pain or tightness of the chest
- Fast heart rate
- Feeling faint or passing out
- Severe or persistent headache
- Difficulty speaking or understanding language
- Feeling confused
- Numbness or weakness in your face, arms, or legs
- Sudden loss or changes in your vision, eye pain, or swelling
What are the possible side effects of Qfitlia?
Qfitlia can cause other serious side effects, including an increase in your blood liver enzymes. Your HCP will do blood tests to check your liver function before and during treatment with Qfitlia
The most common side effects of Qfitlia include viral infection, common cold symptoms, and bacterial infection
These are not all the possible side effects of Qfitlia.
What should I tell my HCP before using Qfitlia?
Tell your HCP about all of your medical conditions, including if you have liver problems, a history of gallbladder disease, are pregnant or plan to become pregnant, or are breastfeeding or plan to breastfeed
Females who are able to become pregnant: Hormonal birth control may increase your risk of blood clots if used during treatment with Qfitlia. Talk to your HCP about effective forms of non-hormonal birth control you can use before starting and during treatment with Qfitlia
Tell your HCP about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements

